In Sept, 2006, the Center for Breast Care brought new technology to assist in the evaluation of our patients with newly diagnosed breast cancer. PEM or Positron Emission Mammography, is an organ specific high resolution PET scanner which will uniquely give our physicians the capability of functional imaging for breast cancer detection. Rather than relying on morphology or the "appearance" of a cancer, PET relies on identifying its metabolism for its diagnosis. By so doing we would anticipate detecting cancers earlier, at a more easily curable stage.
PEM is a nuclear medicine exam which uses intravenous injectable FDG, (fluoro-deoxyglucose), a glucose analog, which accumulates in glucose avid cells. FDG will accumulate in both inflammatory and cancerous states as they have higher metabolic rate than normal cells. This glucose analog is phosphorylated but does not proceed through the entire cellular energy production cycle or Krebs cycle so it maintains its presence within the cell which permits image acquisition.
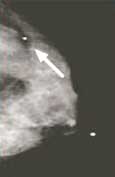
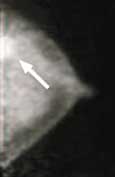
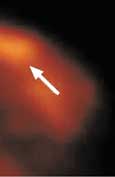
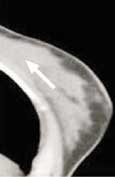
Following a 4 hour fast, serum blood is drown to determine blood glucose level. 10mCi FDG is injected intravenously and imaging is acquired one hour later. Imaging in obtained on the dedicated breast PET in a manner similar to mammography. The PEM detectors are mounted on the 2 compression paddles. 1000 luteitium photodetecting crystals are present on each detector. The breast is imaged, similar to mammography with slight compression to immobilize the breast in the MLO and CC projections. Image acquisition is 10 minutes. One million coincident counts are obtained for image production. Spatial resolution in in the order of 1.5mm, the highest resolution of any biochemical breast imaging modality. This resolution is in the order of size of breast ductal structures.
As the detectors are positioned close to the target organ, image acquisiton is efficient and there is little attenuation of counts so spatial resolution is much improved over whole body PET imaging. Also, as this is a quantitative examination with comparisons of lesion to background FDG concentrations, the exam lends itself to sequential imaging say perhaps in patients with pathologically proven atypia or those involved with prevention drugs.
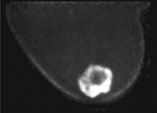
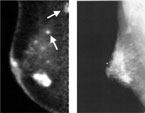

We believe PEM to be of great value in the preoperative identification of non-invasive breast cancer (DCIS). DCIS accounts for 30% of our newly diagnosed patients and the extent of disease is often difficult to quantitate with mammography and MRI. PEM has been reported to have a 91% sensitivity for DCIS which far exceeds all other imaging modalities. DCIS is often seen in the region of invasive breast cancer and if anticipated preoperatively, we may be able to avoid the necessity for re-excision of positive margins obtained at lumpectomy or the possibility of recurrent breast cancer.
As utilization and research proceeds with this new modality, we may be able to combine the information gained from mammography, ultrasound, MRI and PET to formulate a complete evaluation of a patient's extent of disease. This becomes feasible as cross modality correlation is possible particularly with the use of computed aided detection devices. This should permit accurate biopsy guidance from each modality.
Currently we use PEM for pre-operative surgical staging. Patients are actively recruited for participation in the funded study after biopsy proven breast cancer is diagnosed. After informed consent, patients will undergo preoperative breast MRI as well as preoperative PEM and whole body PET scan. Findings from each modality will be compared and reported. The Center for Breast Care is participating in a national multi-institutional trial designed to determine whether PEM improves the way surgeons treat breast cancer. Results of our research will be published as well as any significant new findings, beneficial or otherwise.
Your physicians at the Center for Breast Care attempt to provide you, the patient, with the most current effective imaging and interventional modalities available to detect breast cancer early, accurately and effectively. Should you have questions about this or other services provided, please contact us at 561-955-5000.
Prospective Multicenter Study of the Role of Positron Emission Mammography in Pre-Surgical Planning for Breast Cancer
Funded by Naviscan PET Systems, Inc.,
PI: Kathy Schilling, MD and Medical Director, Breast Imaging and Intervention at the Center for Breast Care, Boca Raton Community Hospital.
In women with newly diagnosed breast cancer who are thought to be candidates for lumpectomy surgery, mammography and clinical breast examination often do not depict the full extent of cancer. As a result, positive margins are common at the initial treatment surgery. Magnetic resonance imaging (MRI) has been shown to be highly sensitive in depicting the full extent of tumor in both the breast with cancer and the opposite breast, and is often used in planning treatment. There are, however, many noncancerous lesions that show up as suspicious on MRI. Positron emission mammography (PEM) is another way to image breast cancer, using a high-resolution PET scanner to image areas where there is increased metabolism of glucose. In preliminary studies, PEM appears to be equivalent to MRI at showing cancer, but it may be less likely to show noncancerous lesions. In this clinical research study, participants will have both a contrast-enhanced MRI of the breast(s) and a PEM study. The MRI is billed to insurance as part of usual care. The PEM study is performed at no charge. The purpose is to determine whether MRI or PEM is better at predicting the actual extent of tumor and at helping surgeons to perform the most appropriate breast surgery at the time of initial diagnosis thereby reducing positive margins and multiple surgeries.
If you are interested in participating in the study, please contact:
