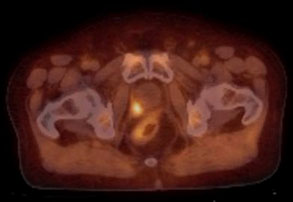
Axumin is an FDA-approved, Medicare-covered Nuclear Medicine scan used for the early detection of recurrent prostate cancer.
Historically, Medical Oncologists and Urologists have relied upon PSA laboratory values, as well as CT examinations and Nuclear Medicine Bone Scans to indicate the presence of recurrent disease. Additionally, until now, the precise location of the recurrent disease has been undetectable until PSA levels climb to at least 10-30 ng/mL.
An Axumin scan is able to detect and localize recurrent disease when PSA levels are significantly lower than even 10 ng/mL, aiding in not only establishing the presence of local vs distant metastatic disease, but enabling formulation of an early treatment plan.
Normal cells rely upon amino acids, but cancer cells utilize amino acids at a significantly greater rate. Axumin scans use F18, a radioactive tracer, linked to an amino acid, and as the radioactive amino acid is taken up by the prostate cancer cells, activity concentrates inside the cancerous cells. When the patient is then placed under a Nuclear Medicine scanner, increased activity is seen at the site of viable, recurrent prostate cancer.